Prices ranges
Consumer blood pressure monitors are available at a wide range of prices, to represent cheap and expensive blood pressure monitors. In the USA, prices can range from $36 to $120. In Europe, prices can range from $20 to $120. These are the prices for stand alone models, upper arm cuff and wrist based models. As for wearables, the non-validated ones go for around 50 to 80 dollars, while the few validated models cost several hundred dollars ($260 for Aktiia Bracelet, $500 for Omron HeartGuide).
The market of blood pressure monitors
Several key players dominate the market of blood pressure monitor. Omron Healthcare Inc., A&D Medical Inc., SunTech Medical, Inc., Koninklijke Philips N.V., Masimo, GE Healthcare (General Electric Company), American Diagnostic Corporation, Beurer GmbH, and SunTech Medical Inc. are among the leading manufacturers. More on the market actors: here, here, here, here, here.
Countries like China, Vietnam, and now increasingly in India produce most blood pressure monitors. Omron, which holds half of the global market for blood pressure monitors, has exported Chinese- and Vietnamese-made devices to India and other countries.
Advanced features found in both cheap and expensive models
Technologically improved blood pressure monitors, regardless of their price, are more convenient and comfortable to use compared to traditional equipment. For instance, some expensive monitors offer features like Bluetooth connectivity, backlit screens, and multiple user profiles. However, these features are not exclusive to expensive models. Some cheaper models also offer similar features, providing the same level of convenience and comfort. Further, expensive blood pressure monitors often come with an irregular-heartbeat detector and can store up to 200 readings for two users. However, cheaper models can also offer these features. Hence, both cheap and expensive blood pressure monitors offer such features.
Features found primarily in the expensive models
- Some expensive blood pressure monitors come with a voice guidance feature, allowing users to measure their blood pressure in about 40 seconds just by pressing the “Start” button.
- Some expensive blood pressure monitors come with a cuff recognition function, reducing the measurement error.
- Some expensive blood pressure monitors come with a large backlit screen, making health parameters clearly visible.
- Some expensive blood pressure monitors are wireless and portable.
Advice to buyers
The best time to buy blood pressure monitors is between May and December. This is likely due to seasonal sales and discounts offered by retailers, they cover both cheap and expensive blood pressure monitors.
Validation: this is what costs most
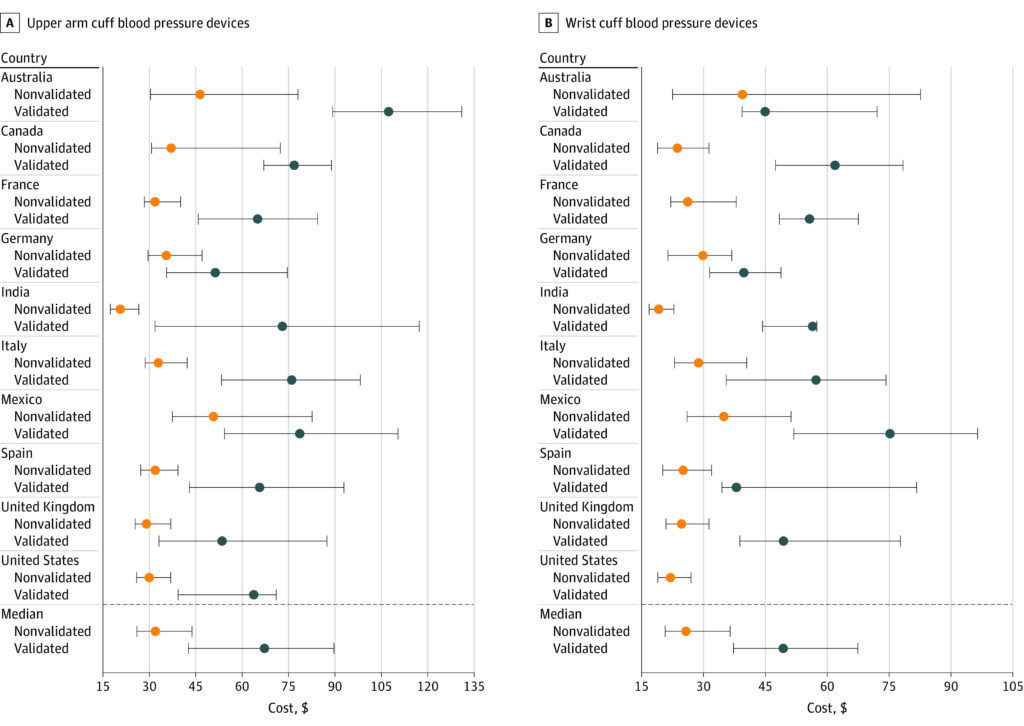
It is the cost of validation that adds to the price of blood pressure monitors. On average, it adds 40-46% to the price. Here is the data for upper cuff arm and for wrist based devices, in 2021 prices, from the featured image above:
Upper Arm Cuff Blood Pressure Devices: Difference Between Cheap and Expensive Blood Pressure Monitors
Australia: Nonvalidated – around $80, Validated – around $100
Canada: Nonvalidated – around $75, Validated – around $125
France: Nonvalidated – around $70, Validated – around $125
Germany: Nonvalidated – around $60, Validated – around $110
India: Nonvalidated – around $20, Validated – around $40
Italy: Nonvalidated – around $60, Validated – around $110
Mexico: Nonvalidated – around $40, Validated – around $80
Spain: Nonvalidated – around $60, Validated – around $100
United Kingdom: Nonvalidated – around $60, Validated – around $100
United States: Nonvalidated – around $50, Validated – around $80
Wrist Cuff Blood Pressure Devices: Difference between cheap and expensive blood pressure monitors
Australia: Nonvalidated – around $50, Validated – around $80
Canada: Nonvalidated – around $50, Validated – around $80
France: Nonvalidated – around $40, Validated – around $80
Germany: Nonvalidated – around $40, Validated – around $70
India: Nonvalidated – around $20, Validated – around $40
Italy: Nonvalidated – around $40, Validated – around $70
Mexico: Nonvalidated – around $30, Validated – around $60
Spain: Nonvalidated – around $40, Validated – around $70
United Kingdom: Nonvalidated – around $40, Validated – around $70
United States: Nonvalidated – around $30, Validated – around $60
Hence, the major difference lies in the clinical validation of the monitors. Expensive monitors from well-known brands are almost all clinically validated, having been extensively tested and approved for use. This is not always the case with cheaper monitors.
FDA clearance is not equal to clinical validation
In the United States, it is not legal to market or sell a medical device, including a blood pressure monitor, without it being registered, cleared, or approved. It is the the Food and Drug Administration (FDA) that does it. The FDA regulates the sale of medical device products and monitors their safety.
For a blood pressure monitor to be legally sold in the U.S., the manufacturer must seek approval from the FDA by presenting evidence that the device is reasonably safe and effective for a particular use. This process often involves submitting a “premarket notification submission” or 510(k) to the FDA. Once the FDA declares that a new medical device is substantially equivalent to a predicate (a device that is already FDA-cleared or -approved), it is “cleared,” and can be marketed and sold in the U.S.
However, FDA clearance does not necessarily represent the accuracy of the device. A substantial number of home blood pressure devices on the market are not validated, and the government has no enforcement division to prohibit selling these devices.
Therefore, while it is illegal to sell a non-FDA approved/cleared blood pressure monitor in the USA, it seems to be legal to market and sell blood pressure monitors with no clinical validation. The presence of FDA approval or clearance does not guarantee the accuracy of the device.
Accuracy of cheap vs expensive blood pressure monitors
Both cheap and expensive monitors can offer a wide range of features and high accuracy. Available studies state a measuring accuracy of ±3mmHg for static pressure. Only in the lowest price zone, differences become noticeable. Thus, in the UK, monitors costing more than £10 were found to be more accurate than those costing less.
In my personal experience, $60 smartwatch that I bought some 5 years ago at a major Chinese marketplace, gave outrageously wrong results, with most significant and irregular, sudden deviations to a clinically validated device. A cheap upper arm cuff based device that I used for a year gave wrong, but persistently wrong results, it showed 10-20mmHg lower readings than a clinically validated stand alone blood pressure monitor.
Most such manufacturers claim at their websites that their blood pressure smartwatches are not medical devices, the results of measurements should not be used for diagnostics, but only for reference. Whatever the latter may mean.
Prevalence of non-validated devices
The JAMA-published 2022 study examined several thousand blood pressure measuring devices. Devices listed were from companies that distribute across Asia, Europe, Africa, Oceania, North America, and South America, as well as by e-commerce. The study found that only 8.8% blood pressure monitors in use were clinically validated, 11.1% were equivalent to be clinically validated. There was no evidence of validation 76.3%.
Among upper arm cuff devices 10.0% were validated, 13.2% were equivalent, and there was no evidence of validation for 73.0%. Wrist-based devices: only 5.6% were validated, further 5.5% were equivalent, there was no evidence of validation for 85.0%.
Another study examining the availability, cost, and consumer ratings of blood pressure–measuring devices sold online in 10 countries, including the United States, 79% of upper arm and 83% of wrist cuff devices were nonvalidated.
Yet another study found that around 8 in 10 of the best-selling blood pressure monitors online have not been validated for accuracy.