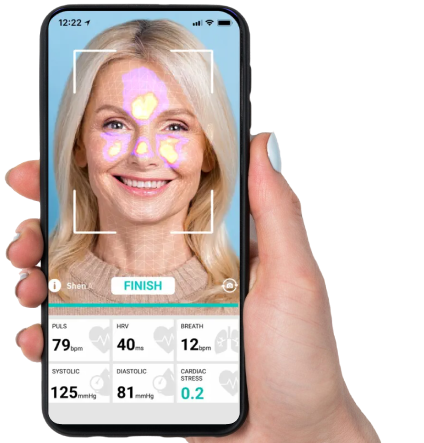
MarinHealth‘s interventional cardiologists are renal denervation pioneers. They have launched a groundbreaking treatment for patients with poorly controlled blood pressure, utilizing the newly FDA-approved Symplicity Spyral renal denervation system. This innovative procedure, the Symplicity Spyral renal denervation, is a minimally invasive approach targeting overactive nerves near the kidneys. The latter are often implicated in high blood pressure.
CEO is enthusiastic
David Klein, MD, CEO of MarinHealth, expressed enthusiasm about being the first on the West Coast to offer this cutting-edge treatment. His team made the procedure possible through the generosity of Reta and Bill Haynes and their family foundation, offering patients a transformative solution for managing their blood pressure.
How Symplicity Spyral Renal denervation Works
The Symplicity Spyral renal denervation procedure stands out for its clinical efficacy in reducing high blood pressure, which is crucial in lowering associated health risks. The process involves the insertion of a thin tube into the artery leading to the kidney. Here the renal denervation pioneers administer energy to calm the hyperactive nerves. They do it without leaving any implant behind, enhancing patient comfort and recovery.
The Provider’s Opinion
Dr. Robert Sperling, an interventional cardiologist at MarinHealth, noted the difficulties many patients face in controlling their blood pressure. Further, he emphasized that lifestyle changes and medication are primary treatments. However they often fall short.
Dr. Robert Sperling, an interventional cardiologist at MarinHealth:
“Renal denervation can significantly reduce blood pressure through a single, minimally invasive procedure, eliminating the need for a permanent implant”
This treatment offers a new avenue for those struggling with blood pressure management. It marks a significant advancement in the field of cardiology and patient care.
Key Facts on Renal Denervation (RDN) in the United States
- Emerging Clinical Procedure: Transcatheter RDN is an emerging clinical procedure for treating hypertension. Initially, the first randomized sham-controlled trial, SYMPLICITY HTN-3, did not show significantly lower blood pressure compared with sham treatment. However, subsequent studies demonstrated the potential importance of more distal and branch renal artery radiofrequency ablation. Newer trials with second-generation radiofrequency ablation systems showed that RDN significantly reduced blood pressure compared to sham treatment in both medicated and unmedicated patients.
- Efficacy in Lowering Blood Pressure: The RADIANCE II Pivotal trial involved 224 patients. It demonstrated the added efficacy of RDN in patients on fewer anti-hypertensive medications. This trial reported a mean reduction in daytime ambulatory systolic blood pressure (SBP) by 7.9 mmHg. Subsequently, it was compared to 1.8 mmHg in the sham group. Long-term data from the SYMPLICITY HTN-3 trial showed a sustained reduction in office SBP over three years. These results align with the Global Symplicity Registry’s findings, which noted a significant reduction in blood pressure in patients treated with RDN.
- Safety Profile: So far, the safety profile of RDN has been excellent. A meta-analysis including 5,769 patients across 50 RDN studies reported only a 0.45% rate of renal artery stenosis or dissection. This suggests that RDN is a safe procedure with a low incidence of serious complications.