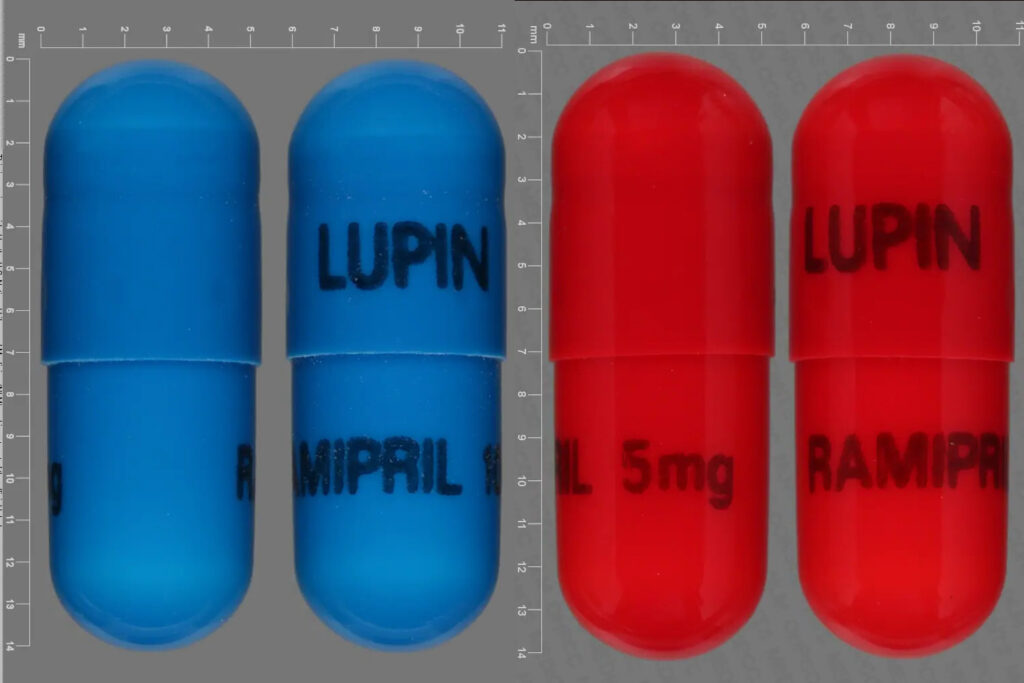
600,000 bottles of a popular blood pressure drug Ramipril are recalled by its manufacturer, Indian drugmaker Lupin.
Lupin recalled the high blood pressure and heart failure drug Ramipril due to the presence of an active pharmaceutical ingredient (API). It came from an unapproved vendor. Ramipril was sold nationwide in the U.S. by 30 wholesalers or distributors.
The voluntary recall was issued according to three separate notifications posted to the FDA’s website. In total, 616,506 bottles of ramipril are being recalled.
Ramipril is used to treat high blood pressure (hypertension) and heart failure, and it’s often prescribed after heart attacks.
The recall is classified as a Class II effort, which is issued when a product has a low chance of causing serious injuries or death. The recalled products were manufactured by Lupin at its facility in Goa, India.
News of the Lupin recall comes just weeks after another similar incident. The U.S. unit of Indian drugmaker Dr. Reddy’s Laboratories recalled 331,590 bottles of cinacalcet tablets. It is used to treat hyperparathyroidism. The reason for the recall was the presence of a known carcinogen. Dr. Reddy’s also issued three separate recalls for drugs that were distributed nationwide.
Two years ago Lupin suspended production of drugs bound for the U.S. The drugs were produced at Tarapur in Maharashtra, an active pharmaceutical ingredient plant in India.
The FDA sent a warning letter to the company in the wake of an inspection. The inspector found the production facility failed to establish proper cleaning protocols. The company also failed to set up in-process samples and controls.